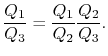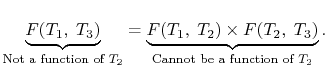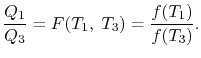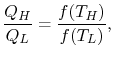BONDS IN SOLID
DEFINE:-
A solid consists of billions of atoms closely packed and held together by strong mutual or interatomic forces of attraction. The attraction and repulsion forces that tend to hold adjacent atoms at a certain spacing to keep the balance between the opposing forces are known as bonds. This process of holding the atoms together is known as atoms. These bonds between atoms make it possible for them to combine in a large mass to form a solid.
The mechanical, physical, electrical and other properties of solids are influenced by the bonds. Therefore, different solids differ in their properties.
Bonds formed between atoms of solids are known as interatomic bonds because they are formed either by transfer of one or more electrons from one atom to another or by sharing of electrons between the atoms.
CLASSIFICATION OF BONDING IN SOLIDS:-
#Various types of bonds found in solids:_
(I) Ionic bond,
(II) Covalent bond,
(III) Metallic bond,
(IV) Van der waal &molecular bond
IONIC BOND:-
Properties of ionic Bonding __
(a) Ionic bonds are generally crystalline in structure, in which ions of one type are surrounded by ions of other type in a systematic way.
(b) These bonds are fair strong.
(c) Due to high binding energies, the ionic crystals have high melting and boiling point.
(d) Poor conductor of heat and electricity as compared to original metals.
(e) Ionic crystals are highly soluble in ionizing solvents such as water and polar solvent like NH3.
(f) They are transparent